Terri Coles | Daily Brew | October 5, 2016 | Yahoo News
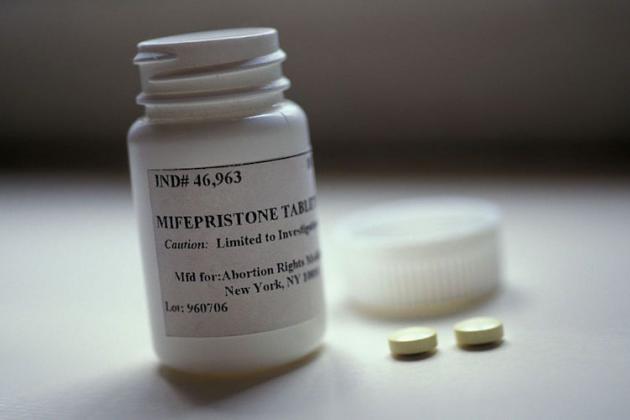
An upcoming cost-effectiveness review for mifepristone, otherwise known as the abortion pill, could lead to provincial funding for the medication and widen access to the drug for those seeking it.
But the move would still leave up to one-third of Canadian women without coverage for medical abortion through either the drug formulary or private insurance, says Sandeep Prasad, executive director of Action Canada for Sexual Health and Rights.
“We’re not going to see take up of this method of medical abortion amongst that segment that doesn’t have access to drugs in the formulary or private insurance until there’s cross coverage for them as well because it’s going to cost nearly $300,” Prasad tells Yahoo Canada News.
And the move to resubmit the drug widely used in other countries after a cost review further delays the introduction of a medication that has already undergone a long and arduous review process, according to Joyce Arthur, executive director of Abortion Rights Coalition of Canada (ARCC).
“It’s indicative of the whole process so far,” Arthur tells Yahoo Canada News. “It’s just disappointing and I do think there’s a pattern that’s been shown by Health Canada.”
Last month, the ARCC issued a statement urging the federal government to intervene in the process to ensure people have access to and coverage of mifepristone.
Extended review process
Celopharma Inc., the Canadian distributor of mifepristone (sold as Mifegymiso), will complete a cost-effectiveness review and resubmit its application to the Common Drug Review, which advises the provinces and territories (excluding Quebec) on which drugs they should reimburse for qualified patients, the Globe and Mail reports Tuesday.
The Canadian Agency for Drugs and Technologies in Health (CADTH) is currently in the process of completing the Common Drug Review for Mifegymiso that will ultimately allow it to be added to each province and territory’s public drug plan formularies, Health Canada spokeswoman Maryse Durette tells Yahoo Canada News in an email.
“Once a drug is approved (deemed safe and efficient) for distribution/sale in Canada, the manufacturer is responsible for its availability and its cost,” Durette says. “Health Canada does not have a role in the pricing or the availability. Ultimately, it is up to the company to advise Health Canada when the product is available for prescribing.”
A submission for Mifegymiso hasn’t happened yet, according to Andrea Tiwari of theCanadian Agency for Drugs and Technology in Health. The CADTH runs the Common Drug Review process that determines if a drug is eligible for public reimbursement.
“When we do receive drug submissions, our aim is to complete the drug review within 180 days, as mentioned in our procedures document,” Tiwari tells Yahoo Canada News. “We cannot comment on when the drug could be added to public formularies as those decisions are made by individual jurisdictions.”
In July, Celopharma withdrew its Common Drug Review application because it could not afford the $72,000 review cost, the Globe and Mail reports. Celopharma Inc. did not reply to a request for comment.
It remains unclear when the drug, approved by Health Canada nearly a year ago, will be available to Canadians as there is no current information showing that mifepristone is undergoing a cost review, but Arthur says it seems safe to assume it won’t be available before next spring.
The long review process for mifepristone has also added to the out-of-pocket cost of the drug, which is much lower in Europe and the United States, Arthur says.
Access remains limited
Adding mifepristone to provincial and territorial drug schedules has the potential to widen access to the medication, which induces an abortion during the early stages of pregnancy. But that would leave out those who do not have access to drugs on their province or territory’s formulary, which covers those receiving some form of social assistance and those lacking access through a private insurer.
Action Canada is researching mifepristone and private insurance coverage for the drug in Canada and it appears private insurers aren’t necessarily going to include it in their standard plans, Prasad says.
“We’ve received one response from one benefit provider, a major one, and they have said that Mifegymiso will not be covered,” Prasad notes. Employers would have the option to add the drug to plans offered by the unnamed insurer, but it would not be standard, he adds.
If people lack coverage for the drug, those seeking an abortion will have to either opt for a surgical abortion, which has mixed availability across the country but is covered by provincial and territorial health plans, or pay for mifepristone out of pocket at a cost of about $300.
That doesn’t make sense strictly from a cost-benefit analysis, Prasad says, considering that a surgical abortion costs three to four times more than a medical one.
“This should make it absolutely clear that we need universal health coverage of medical abortion using Mifegymiso,” Prasad asserts. “Medical abortion needs to be covered just like surgical.”